Fluoride toothpaste: benefits and harms, effects on teeth
The life of a modern person is full of myths convenient for manufacturers of goods. To mislead people with semi-truthful statements has become commonplace. One of these "stumbling blocks" is fluoride, which is part of toothpaste, and reasoning about its benefits and harms.
The companies that produce fluoride pastes speak of them as a panacea. Their opponents argue that in large quantities, the element is life threatening. The consumer, on the other hand, needs to have an open and competent look at the situation and draw appropriate conclusions for himself.
Content
The effect of fluoride on human teeth
In 1990, University of California students performed a social experiment. They spread alarming information that the water is contaminated with dihydrogen monooxide. Their statement produced the effect of a “bursting bomb” among the public.
In fact, dihydrogen monoxide is one of the chemical names for ordinary water (H₂O formula). A joking but very visual experience showed how gullible people who are ignorant of science can be, and how the lack of basic knowledge leads to an increase in panic. Thanks to the experiment, the term “Zonerism” appeared, when facts are used to lead to false conclusions. It is used by manufacturers of toothpastes.
Ardent opponents appeal that in nature pure fluorine is a poisonous gas. But it is introduced into the composition of oral hygiene products in the form of fluoride compounds having completely different properties.
It is worth agreeing that in large quantities fluoride is really harmful. But paraphrasing the words of Paracelsus, we can safely say that "all the medicine and all the poison, the difference is only in quantity." And in order to “accumulate” an element in a dangerous concentration, it must be swallowed. It is unlikely that anyone does this with toothpaste.
That is why before making a conclusion about the benefits and harms of toothpaste with fluoride in the composition, you need to find out what fluoride is really good or bad.
Beneficial fluoride
The positive effect of fluoride on teeth was discovered more than 100 years ago, and already in 1914, the Americans launched the production of fluorinated toothpastes. The anticariogenic effect of the element has been proven by numerous studies. His a beneficial effect is expressed in an increase in enamel resistance to destruction from the vital products of microorganisms living in the oral cavity by 35–40%. Fluoride in various compounds is still used in toothpastes, and is this not an indicator that it is necessary for healthy teeth.
The main quality of fluorine, due to which it is needed and used in toothpaste, is its anticariogenic ability. It is characterized by the following features:
- Enamel Strengthening. Fluorine, dissolved in different salts, when it reacts with saliva releases free ions. Which, in turn, react with calcium compounds. When bound, they turn into the element fluorapatite, which is strong and resistant to microorganisms. It is he who is engaged in "patching" of damaged areas of enamel. The process is called remineralization. Fluoride pastes inhibit the rate of caries development better than others.
- Bactericidal effect. Fluorine, being a natural antiseptic, has an antibacterial effect on all types of cariogenic microorganisms in the oral cavity.
- The fight against dental deposits. After using fluoride pastes, food residues do not stick to the walls of the teeth; accordingly, stones do not form.
- Strengthening remineralization ability of saliva. Active salivation is very important for saturation of enamel with calcium and fluorine minerals. Pastes increase the working activity of the salivary glands, thereby compensating for the demineralization process.
- Participation in metabolic processes. The use of fluoride in microdoses is useful for establishing metabolism. This phenomenon is important for women during pregnancy, as it contributes to the full formation of the fetus.
Despite the fact that the element is characterized by a large number of positive qualities, it is worth noting its disadvantages.
Harmful fluoride
In large quantities, fluorine can be not only harmful, but also dangerous to health. It is distinguished by its insidious quality of accumulating in the body, but it is almost impossible to remove it. In the event of a glut, it will spread its toxicity to the body and poison it.
The element affects the appearance of joint mobility and the appearance of bone fragility. A complete list of what fluorine is dangerous, including in the composition of toothpaste, for an adult, looks like this:
- Disruption of the hormonal background. Fluorine ions can accumulate over time in the thyroid gland, which affects the production of hormones. Specifically, the synthesis of therioids and toastosterone is changing, which is fraught with the development of diseases of the reproductive system in girls and the sexual - in boys.
-
Fluorine ions in large quantities provoke the development of a carcinogenic reaction, which increases the risk of developing cancer.
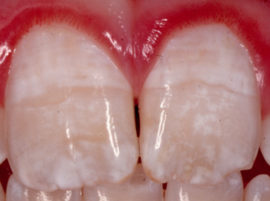
- Fluorosis develops. The problem is from the category of dental, but it is laid with intrauterine development, if fluoride is present with a plus sign in the diet of the expectant mother. The disease proceeds in different ways. In the first form, the enamel becomes spotty, with time the spots turn from yellowish to brown - the enamel is completely destroyed. In the second case, skeletal bone tissue becomes fragile, which increases the risk of fractures and inhibits the recovery process.
- There is a neurotoxic effect on the children's body, therefore, fluoride-containing pastes at this age should be used limitedly. Children “poisoned” with an excess of fluorine are united by such differences: problems with the development of speech and memorization.
Fluoride in toothpaste can be harmful, even if it contains a minimal amount. It’s just that it can become the “last straw” with an excess of an element in the body, and then a negative impact on all life support systems cannot be avoided.
Fluoride Compounds in Toothpastes
To understand whether fluoride is useful in toothpastes, you need to find out what its quantitative content is, and what kind of compound it is represented. Since in pure form it is a gaseous substance, it is introduced into the composition of the paste in the form of soluble salts. The difference between them is obvious, therefore, the influence is not the same.
Sodium fluoride in a toothpaste breaks down into ions in the shortest possible time, releasing active fluoride. The substance has a high remineralizing ability, which directly affects the increase in the anticariogenic effect. Sodium fluoride is preferred for the manufacture of children's toothpastes. Since the kids do not want to spend a lot of time on oral hygiene, the result should appear in a short period of time, which is provided by the paste with this compound.
The sodium monofluorophosphate salts in the toothpaste dissociate into ions quite slowly. Such a paste will have to brush your teeth for at least three minutes, which in principle is not critical, but completely unsuitable for children. To use paste with monofluorophosphate, an adult patient is needed.
Aminofluoride in toothpaste has another name - olaflur. This fluoride compound is by far the most progressive.A paste with aminofluoride has the highest remineralizing property and, in addition to providing an anti-carious effect, creates a kind of protective film on the tooth enamel, as if sealing it. Aminofluoride allows the mineral saturation process to last as long as possible.
The tin fluoride compound has been actively used in toothpaste from the very beginning. Salt has a high remineralizing ability, which is its positive quality. The negative properties of tin fluoride are manifested in the fact that the compound unevenly whitens the tooth enamel, highlighting some of its areas more strongly. And over time, paradoxically, they begin to darken. Most companies abandoned the use of tin fluoride, replacing it with monofluorophosphate or other salts.
In addition to the above salts, the composition of the “cosmetics” for the teeth may include Sodium Monofluorophosphate, surfactants or SLS, saccharin, cocamidopropyl betaine, chlorhexine and much more.
Fluoride Toothpastes for Children
Conscious parents seek to control the fluoride content in their children's toothpastes. Someone, believing that at the "tender" age, it is both harmful and dangerous, replaces it with a calcium product. Others, realizing that it is useful for good condition of tooth enamel, seek to limit its influence by choosing products with a regulated fluoride content.
Manufacturers adhere to the following standards for the ratio of age and fluoride content in the paste:
- 1–4 years - up to 200 ppm fluorides;
- 4-8 years - up to 500 ppm fluorides;
- 8 or more years (as well as for adults) - up to 1400 ppm of fluoride.
There is a clear trend that the older the child, the more fluoride-rich toothpaste he can use. This happens because the older the baby, the less likely it is to swallow this delicious substance.
The best cosmetics for brushing your teeth is the one that suits the child both by age and by solving a specific problem. If the teeth are susceptible to caries damage, then fluoride-containing paste cannot be dispensed with, and in the case of a normal condition of the teeth, calcium can be preferred.
The ratio of the benefits and harms of fluoride for the teeth of young children is ambiguous, so you need to consult a dentist for advice on this issue.
Where else is fluoride
When a person thinks about how the fluorine contained in the paste affects his teeth, we can say that he is "not digging there." People get the largest amount of mineral from tap water, but in some areas there is a deficiency of fluoride, for example, in Moscow.
Many people think that if there is little fluoride in the water, then you can replenish the supply only using fluoride-containing paste. But the unrest is in vain, since the mineral is contained in a large number of ordinary products:
- sea and freshwater fish;
- seafood;
- all types of tea;
- apples
- grapefruits;
- bow;
- spinach;
- potatoes;
- pumpkin;
- offal (liver);
- milk products;
- cereals (buckwheat, oatmeal);
- cereals (rice);
- honey.
What is fluoride for toothpaste for?
Those who are wondering why fluoride is in toothpaste, if the necessary amount can be obtained by organizing a full-fledged diet, can hear this answer from the dentist: the difference in the method and place of fluorine activation in the body. You can eat as many apples as you like, but it won’t save you from caries. And brushing your teeth will not benefit the bones of the skeleton. Both the first and second options are useful, but each is used for a specific purpose and in moderation.
Tips for oral hygiene
Instead of assessing the harm of toothpaste to the body and chasing pseudo-naturalness, it is better to fully and correctly organize oral hygiene. A few tips:
- hygiene procedures should be carried out regularly;
- children are taught to brush their teeth as early as possible, at first without toothpaste;
- cleaning with soda is permissible no more than 1 time per week;
- rinsing the mouth with elixirs, using dental floss is an excellent prevention of caries, periodontitis and calculus deposits;
- a toothbrush changes at least once a year;
- you need to brush your teeth 2 times a day - in the morning and at night.
The decision about which toothpaste is better - with fluoride or without fluoride - each one makes for himself. Nevertheless, it will be useful to consult with your dentist about this.